Showing 5651–5700 of 25168 results
-

COLUMN C 10/40
$424.12 Add to cart View Product DetailsCOLUMN C 10/40
-

COLUMN C 16/100
$522.59 Add to cart View Product DetailsCOLUMN C 16/100
-

COLUMN C 16/20
$455.45 Add to cart View Product DetailsCOLUMN C 16/20
-

COLUMN C 16/40
$495.74 Add to cart View Product DetailsCOLUMN C 16/40
-

COLUMN C 16/70
$522.59 Add to cart View Product DetailsCOLUMN C 16/70
-

COLUMN C 26/100
$757.59 Add to cart View Product DetailsCOLUMN C 26/100
-
COLUMN C 26/40
$560.64 Add to cart View Product DetailsCOLUMN C 26/40
-

COLUMN C 26/70
$605.40 Add to cart View Product DetailsCOLUMN C 26/70
-

COLUMN PD 10
$437.55 Add to cart View Product DetailsCOLUMN PD 10
-

COLUMN PD-10,EMPTY
$297.67 Add to cart View Product DetailsCOLUMN PD-10,EMPTY
-

COLUMN TUBE HiScale 50 – 20
$1,694.23 Add to cart View Product DetailsCOLUMN TUBE HiScale 50 – 20
-

COMB, 10 WELL, 1.5MM
$160.35 Add to cart View Product DetailsCOMB, 10 WELL, 1.5MM
-

COMB, 15WELL, 1.0MM
$160.35 Add to cart View Product DetailsCOMB, 15WELL, 1.0MM
-

COMB, SPINELESS, 10 WELL, .75
$93.34 Add to cart View Product DetailsCOMB, SPINELESS, 10 WELL, .75
-

COMB, SPINELESS, 10 WELL, 1.5
$92.95 Add to cart View Product DetailsCOMB, SPINELESS, 10 WELL, 1.5
-

COMB, SPINELESS, 10 WELL,1.0MM
$93.34 Add to cart View Product DetailsCOMB, SPINELESS, 10 WELL,1.0MM
-

COMB, SPINELESS, 15 WELL, 1.5
$92.95 Add to cart View Product DetailsCOMB, SPINELESS, 15 WELL, 1.5
-
COMB, SPINELESS, 15 WELL,1.0MM
$92.95 Add to cart View Product DetailsCOMB, SPINELESS, 15 WELL,1.0MM
-

COMB, SPINELESS,15 W,0.75
$93.34 Add to cart View Product DetailsCOMB, SPINELESS,15 W,0.75
-

Combination OF FS002K10 and FS008K10
$5,908.94 Add to cart View Product DetailsCombination OF FS002K10 and FS008K10
-

Combretastatin A4
$88.14 Add to cart View Product DetailsCombretastatin A4
-

Combretastatin A4
$146.60 Add to cart View Product DetailsCombretastatin A4
-

Complement C1q Protein
$556.96 Add to cart View Product DetailsComplement C1Q Protein
-

Compound 48/80
$133.45 Add to cart View Product DetailsCompound 48/80
-

Compound 48/80
$267.66 Add to cart View Product DetailsCompound 48/80
-

Compound 48/80
$1,082.25 Add to cart View Product DetailsCompound 48/80
-

Compound E
$142.77 Add to cart View Product DetailsCompound E
-

Compound E
$1,231.56 Add to cart View Product DetailsCompound E
1,070.92 -

CON-A SEPHAROSE 4B 100 ML
$1,256.68 Add to cart View Product DetailsCON-A SEPHAROSE 4B 100 ML
-

CON-A SEPHAROSE 4B 5 ML
$120.86 Add to cart View Product DetailsCON-A SEPHAROSE 4B 5 ML
-

Concanamycin A (High purity)
$133.35 Add to cart View Product DetailsConcanamycin A (High purity)
-

Concanamycin A (High purity)
$983.20 Add to cart View Product DetailsConcanamycin A (High purity)
-

Concanavalin A
$747.11 Add to cart View Product DetailsConcanavalin A
-

Concanavalin A
$66.57 Add to cart View Product DetailsConcanavalin A
-

Concanavalin A
$137.45 Add to cart View Product DetailsConcanavalin A
-

Concanavalin A
$223.01 Add to cart View Product DetailsConcanavalin A
-

Concanavalin A
$295.83 Add to cart View Product DetailsConcanavalin A
-

Concanavalin A, FITC conjugated
$38.86 Add to cart View Product DetailsConcanavalin A, Fitc Conjugated
-

Concanavalin A, FITC conjugated
$98.61 Add to cart View Product DetailsConcanavalin A, Fitc Conjugated
-

Concanavalin A, highly purified
$138.48 Add to cart View Product DetailsPure Canavalia ensiformis lectin (Con A) from Jackbean. Isolated by affinity chromatography on cross-linked dextran. Con A exists as a dimer below pH 5.0 and and at pH >7 it exists as a tetramer. Con-A is not a glycoprotein. The monomeric molecular weight of Con-A is 25,500. Con-A does not contain cysteine residues. Unlike most other lectins, Con-A is a metalloprotein and requires a transition metal ion, such as manganese, plus calcium ions for binding. Lectins are proteins or glycoproteins of non-immune origin that agglutinate cells and/or precipitate complex carbohydrates. Lectins are capable of binding glycoproteins even in presence of various detergents. The agglutination activity of these highly specific carbohydrate-binding molecules is usually inhibited by a simple monosaccharide, but for some lectins, di, tri, and even polysaccharides are required.
-

Concanavalin A, highly purified
$222.61 Add to cart View Product DetailsPure Canavalia ensiformis lectin (Con A) from Jackbean. Isolated by affinity chromatography on cross-linked dextran. Con A exists as a dimer below pH 5.0 and and at pH >7 it exists as a tetramer. Con-A is not a glycoprotein. The monomeric molecular weight of Con-A is 25,500. Con-A does not contain cysteine residues. Unlike most other lectins, Con-A is a metalloprotein and requires a transition metal ion, such as manganese, plus calcium ions for binding. Lectins are proteins or glycoproteins of non-immune origin that agglutinate cells and/or precipitate complex carbohydrates. Lectins are capable of binding glycoproteins even in presence of various detergents. The agglutination activity of these highly specific carbohydrate-binding molecules is usually inhibited by a simple monosaccharide, but for some lectins, di, tri, and even polysaccharides are required.
-

Concanavalin A, highly purified
$45.15 Add to cart View Product DetailsPure Canavalia ensiformis lectin (Con A) from Jackbean. Isolated by affinity chromatography on cross-linked dextran. Con A exists as a dimer below pH 5.0 and and at pH >7 it exists as a tetramer. Con-A is not a glycoprotein. The monomeric molecular weight of Con-A is 25,500. Con-A does not contain cysteine residues. Unlike most other lectins, Con-A is a metalloprotein and requires a transition metal ion, such as manganese, plus calcium ions for binding. Lectins are proteins or glycoproteins of non-immune origin that agglutinate cells and/or precipitate complex carbohydrates. Lectins are capable of binding glycoproteins even in presence of various detergents. The agglutination activity of these highly specific carbohydrate-binding molecules is usually inhibited by a simple monosaccharide, but for some lectins, di, tri, and even polysaccharides are required.
-

Concanavalin A, highly purified
$449.51 Add to cart View Product DetailsPure Canavalia ensiformis lectin (Con A) from Jackbean. Isolated by affinity chromatography on cross-linked dextran. Con A exists as a dimer below pH 5.0 and and at pH >7 it exists as a tetramer. Con-A is not a glycoprotein. The monomeric molecular weight of Con-A is 25,500. Con-A does not contain cysteine residues. Unlike most other lectins, Con-A is a metalloprotein and requires a transition metal ion, such as manganese, plus calcium ions for binding. Lectins are proteins or glycoproteins of non-immune origin that agglutinate cells and/or precipitate complex carbohydrates. Lectins are capable of binding glycoproteins even in presence of various detergents. The agglutination activity of these highly specific carbohydrate-binding molecules is usually inhibited by a simple monosaccharide, but for some lectins, di, tri, and even polysaccharides are required.
-

Conduritol B epoxide
$124.91 Add to cart View Product DetailsConduritol B Epoxide
-

Conduritol B epoxide
$424.91 Add to cart View Product DetailsConduritol B Epoxide
-
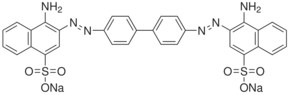
Congo Red
$19.42 Add to cart View Product DetailsCongo Red
-

Congo Red
$54.00 Add to cart View Product DetailsCongo Red
-

Congo Red
$221.92 Add to cart View Product DetailsCongo Red
-

Congo Red
$35.27 Add to cart View Product DetailsCongo Red
-

Congo Red
$133.65 Add to cart View Product DetailsCongo Red






