28822-58-4
Showing all 6 results
-
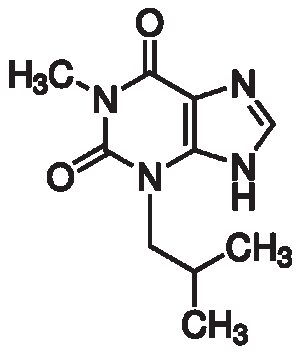
3-Isobutyl-1-methyl xanthine
$47.54 Add to cart View Product DetailsIBMX has been shown to be a potent, non-specific inhibitor of adenosine 3′,5′-cyclic monophosphate phosphodiesterase (cAMP PDE)4, significantly more effective than theophylline. Also inhibits cGMP phosphodiesterases. IBMX inhibits cyclic nucleotide PDE with subsequent inhibition of cyclic nucleotide hydrolysis, resulting in accumulation of cyclic AMP and guanosine 3′,5′-cyclic monophosphate.
-

3-Isobutyl-1-methyl xanthine
$74.72 Add to cart View Product DetailsIBMX has been shown to be a potent, non-specific inhibitor of adenosine 3′,5′-cyclic monophosphate phosphodiesterase (cAMP PDE)4, significantly more effective than theophylline. Also inhibits cGMP phosphodiesterases. IBMX inhibits cyclic nucleotide PDE with subsequent inhibition of cyclic nucleotide hydrolysis, resulting in accumulation of cyclic AMP and guanosine 3′,5′-cyclic monophosphate.
-

3-Isobutyl-1-methyl xanthine
$211.34 Add to cart View Product DetailsIBMX has been shown to be a potent, non-specific inhibitor of adenosine 3′,5′-cyclic monophosphate phosphodiesterase (cAMP PDE)4, significantly more effective than theophylline. Also inhibits cGMP phosphodiesterases. IBMX inhibits cyclic nucleotide PDE with subsequent inhibition of cyclic nucleotide hydrolysis, resulting in accumulation of cyclic AMP and guanosine 3′,5′-cyclic monophosphate.
-

3-Isobutyl-1-methyl xanthine
$853.23 Add to cart View Product DetailsIBMX has been shown to be a potent, non-specific inhibitor of adenosine 3′,5′-cyclic monophosphate phosphodiesterase (cAMP PDE)4, significantly more effective than theophylline. Also inhibits cGMP phosphodiesterases. IBMX inhibits cyclic nucleotide PDE with subsequent inhibition of cyclic nucleotide hydrolysis, resulting in accumulation of cyclic AMP and guanosine 3′,5′-cyclic monophosphate.
-

IBMX
$161.75 Add to cart View Product DetailsIBMX
-

IBMX
$256.15 Add to cart View Product DetailsIBMX






