1mg
Showing 7751–7800 of 7910 results
-
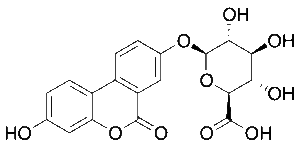
Urolithin A Glucuronide
$150.08 Add to cart View Product DetailsMolecular Formula : C19 H16 O10
-
Urolithin A-2,4,7-d3 Major (>85%)
$133.69 Add to cart View Product DetailsMolecular Formula : C13H5D3O4
-
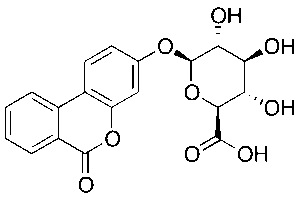
Urolithin B Glucuronide
$181.13 Add to cart View Product DetailsMolecular Formula : C19 H16 O9
-
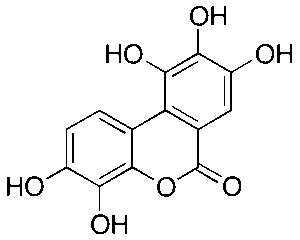
Urolithin M5
$238.05 Add to cart View Product DetailsMolecular Formula : C13 H8 O7
-
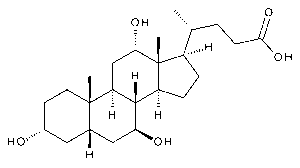
Ursocholic Acid
$113.85 Add to cart View Product DetailsMolecular Formula : C24 H40 O5
-
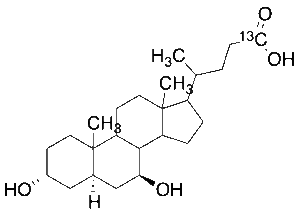
Ursodeoxycholic Acid-24-13C
$75.90 Add to cart View Product DetailsMolecular Formula : No Data Available
-
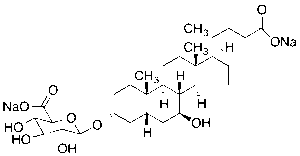
Ursodeoxycholic Acid-3-O-Beta-D-glucuronide Disodium Salt
$130.24 Add to cart View Product DetailsMolecular Formula : C30H46Na2O10
-
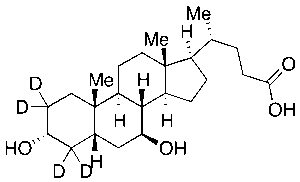
Ursodeoxycholic Acid-d4
$77.63 Add to cart View Product DetailsMolecular Formula : C24 2H4 H36 O4
-

Ursodeoxycholic Acid-d4 (Major)
$65.55 Add to cart View Product DetailsMolecular Formula : C24 2H4 H36 O4
-

Ursodeoxycholic Acid-d5 (Contains d0)
$266.51 Add to cart View Product DetailsMolecular Formula : C24H35D5O4
-
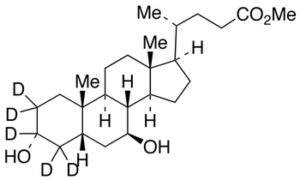
Ursodeoxycholic Acid-d5 Methyl Ester
$154.39 Add to cart View Product DetailsMolecular Formula : C25H37D5O4
-

Uvinul A Plus-d4
$136.28 Add to cart View Product DetailsMolecular Formula : C24H27D4NO4
-

Vaborbactam
$269.96 Add to cart View Product DetailsMolecular Formula : C12H16BNO5S
-

Vaccenic Acid Ethyl-d5 Ester
$78.49 Add to cart View Product DetailsMolecular Formula : C20H33D5O2
-

Valacyclovir-d4, Hydrochloride
$238.91 Add to cart View Product DetailsMolecular Formula : C13H17D4ClN6O4
-

Valdecoxib-13C2,15N
$218.21 Add to cart View Product DetailsMolecular Formula : 13C2 C14 H14 15N N O3 S
-
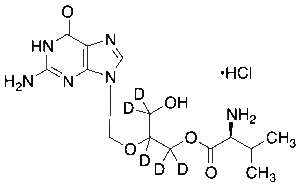
Valganciclovir-d5 Hydrochloride
$271.69 Add to cart View Product DetailsMolecular Formula : C14H18D5ClN6O5
-

Validoxylamine A
$198.38 Add to cart View Product DetailsMolecular Formula : C14H25NO8
-
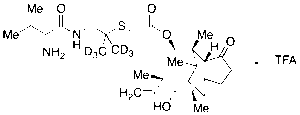
Valnemulin Trifluoroacetic Acid Salt-d6
$1,667.21 Add to cart View Product DetailsMolecular Formula : C33 H47 D6 F3 N2 O7 S
-
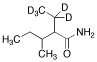
Valnoctamide-d5
$166.46 Add to cart View Product DetailsMolecular Formula : C8H12D5NO
-
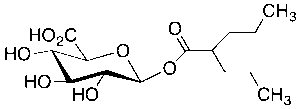
Valproic Acid beta-D-Glucuronide
$209.59 Add to cart View Product DetailsMolecular Formula : C14 H24 O8
-
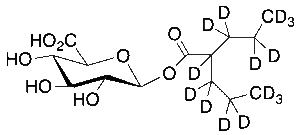
Valproic Acid-d15 Beta-D-Glucuronide
$214.76 Add to cart View Product DetailsMolecular Formula : C14H9D15O8
-
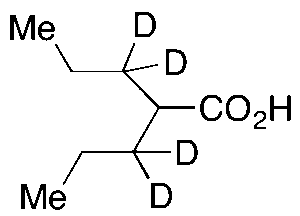
Valproic Acid-d4
$188.89 Add to cart View Product DetailsMolecular Formula : C8 D4 H12 O2
-
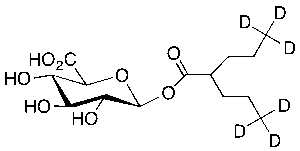
Valproic Acid-d6 Beta-D-Glucuronide
$324.30 Add to cart View Product DetailsMolecular Formula : C14H18D6O8
-

Valrubicin
$55.20 Add to cart View Product DetailsMolecular Formula : C34 H36 F3 N O13
-
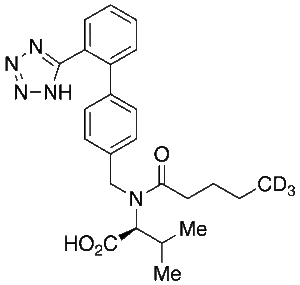
Valsartan-d3
$251.85 Add to cart View Product DetailsMolecular Formula : C24 H26 D3 N5 O3
-

Valsartan-d9 (Major)
Read more View Product DetailsMolecular Formula : C24H20D9N5O3
-

Valspodar
$160.43 Add to cart View Product DetailsMolecular Formula : C63 H111 N11 O12
-

Vanillin-13C6
$320.85 Add to cart View Product DetailsMolecular Formula : 13C6 C2 H8 O3
-
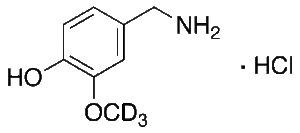
Vanillylamine-d3 Hydrochloride
$132.83 Add to cart View Product DetailsMolecular Formula : C8H9D3ClNO2
-

Vardenafil Dimer
$87.98 Add to cart View Product DetailsMolecular Formula : C38 H46 N10 O8 S2
-

Vardenafil-d5
$210.45 Add to cart View Product DetailsMolecular Formula : C23 2H5 H27 N6 O4 S
-
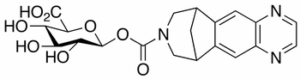
Varenicline Carbamoyl Beta-D-Glucuronide
$219.08 Add to cart View Product DetailsMolecular Formula : C20H21N3O8
-
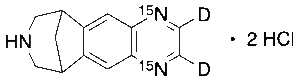
Varenicline-d2,15N2 Dihydrochloride
$274.28 Add to cart View Product DetailsMolecular Formula : C13H13D2Cl2N15N2
-
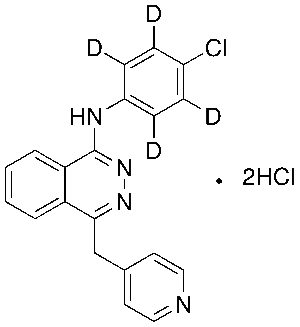
Vatalanib-d4 Dihydrochloride
$166.46 Add to cart View Product DetailsMolecular Formula : C20H13D4Cl3N4
-

Vedaprofen-d3
$210.45 Add to cart View Product DetailsMolecular Formula : C19H19D3O2
-
VEGF-A164, Mouse
$1,470.56 Add to cart View Product DetailsVascular Endothelial Growth Factor (VEGF) was initially purified from media conditioned by normal bovine pituitary folliculo-stellate cells and by a variety of transformed cell lines as a mitogen specific for vascular endothelial cells. It was subsequently found to be identical to an independently discovered vascular permeability factor (VPF), which was previously identified in media conditioned by tumor cell lines based on its ability to increase the permeability of capillary blood vessels. Three mouse cDNA clones, which arise through alternative splicing and which encode mature mouse monomeric VEGF having 120, 164, or 188, amino acids, respectively, have been identified. Two receptor tyrosine kinases (RTKs), Flt-1 and Flk-1 (the mouse homologue of human KDR), both members of the type III subclass of RTKs containing seven immunoglobulin-like repeats in their extracellular domains, have been shown to bind VEGF with high affinity. The roles of the homodimers of KDR, Flt, and the heterodimer of KDR/Flt in VEGF signal transduction remain to be elucidated. In vivo, VEGF has been found to be a potent angiogenesis inducer.
-

VEGF-C, Human
$1,323.94 Add to cart View Product DetailsVascular endothelial growth factor C (VEGF-C) is a member of the platelet-derived growth factor/vascular endothelial growth factor (PDGF/VEGF) family, is active in angiogenesis, lymphangiogenesis and endothelial cell growth and survival, and can also affect the permeability of blood vessels. VEGF-C is expressed in various tissues, however it is not produced in peripheral blood lymphocytes. It forms cell surface-associated non-covalent disulfide linked homodimers, and can bind and activate both VEGFR-2 (flk1) and VEGFR-3 (flt4) receptors. The structure and function of VEGF-C is similar to those of vascular endothelial growth factor D (VEGF-D).
-

VEGF-D, Human
$2,018.25 Add to cart View Product DetailsVascular Endothelial Growth Factor (VEGF)-D, also known as c-Fos-induced growth factor (FIGF), is a member of the PDGF/VEGF growth factor family. It is expressed highly in lung, heart and small intestine, and at lower levels in skeletal muscle, colon and pancreas. It binds to VEGFR-2 and VEGFR-3 receptors and activates downstream signals. VEGF-D is a growth factor active in angiogenesis, lymphangiogenesis and endothelial cell growth. It is involved in many developmental and physiological processes including the formation of venous and lymphatic vascular systems during embryogenesis and the maintenance of differentiated lymphatic endothelium in adults. In tumor pathology, it has been reported to play a role in restructuring of lymphatic channels and regional lymph node metastasis.
-

VEGF-R2 Fc Chimera, Mouse
$1,035.00 Add to cart View Product DetailsVEGF-R2 belongs to a family of proteins called receptor tyrosine kinases. The receptor has three main parts: one part extends out of the cell and binds to VEGF, another spans the cell’s membrane, while the third part is found inside the cell. The current model of VEGF-R2 activation is that VEGF binds to individual VEGF-R2 receptor proteins on the membrane, and brings two of them close enough to form a complex called a dimer. The receptor dimer is activated and initiates signaling within the cell. VEGF-R2 is a receptor tyrosine kinase (RTK) which transduces biochemical signals via lateral dimerization in the plasma membrane. Like most RTKs, VEGF-R2 is composed of an extracellular (EC) domain, a transmembrane (TM) domain, and an intracellular (IC) domain consisting of a kinase domain and sequences required for downstream signaling. The EC domain consists of seven immunoglobulin homology (Ig) domains, termed D1 (at the N-terminus) to D7 (closest to the membrane). VEGF-R2 binds to, and is activated by the ligands VEGF-A, VEGF-E, and a number of processed forms of VEGF-C and VEGF-D. Ligand binding to VEGF-R2 is mediated by Ig-domains 2 and 3 and the linker between D2 and D3.
-

VEGF120, Mouse
$3,187.80 Add to cart View Product DetailsVEGF was initially purified from media conditioned by normal bovine pituitary folliculo-stellate cells and by a variety of transformed cell lines as a mitogen specific for vascular endothelial cells. It was subsequently found to be identical to an independently discovered vascular permeability factor (VPF), which was previously identified in media conditioned by tumor cell lines based on its ability to increase the permeability of capillary blood vessels. Three mouse cDNA clones, which arise through alternative splicing and which encode mature mouse monomeric VEGF having 120, 164, or 188, amino acids, respectively, have been identified. Two receptor tyrosine kinases (RTKs), Flt-1 and Flk-1 (the mouse homologue of human KDR), both members of the type III subclass of RTKs containing seven immunoglobulin-like repeats in their extracellular domains, have been shown to bind VEGF with high affinity. The roles of the homodimers of KDR, Flt, and the heterodimer ofKDR/Flt in VEGF signal transduction remain to be elucidated.In vivo, VEGF has been found to be a potent angiogenesis inducer.
-

VEGF164, Mouse (P. pastoris-expressed)
$1,470.56 Add to cart View Product DetailsVascular Endothelial Growth Factor (VEGF) was initially purified from media conditioned by normal bovine pituitary folliculo-stellate cells and by a variety of transformed cell lines as a mitogen specific for vascular endothelial cells. It was subsequently found to be identical to an independently discovered vascular permeability factor (VPF), which was previously identified in media conditioned by tumor cell lines based on its ability to increase the permeability of capillary blood vessels. Three mouse cDNA clones, which arise through alternative splicing and which encode mature mouse monomeric VEGF having 120, 164, or 188, amino acids, respectively, have been identified. Two receptor tyrosine kinases (RTKs), Flt-1 and Flk-1 (the mouse homologue of human KDR), both members of the type III subclass of RTKs containing seven immunoglobulin-like repeats in their extracellular domains, have been shown to bind VEGF with high affinity. The roles of the homodimers of KDR, Flt, and the heterodimer of KDR/Flt in VEGF signal transduction remain to be elucidated. In vivo, VEGF has been found to be a potent angiogenesis inducer.
-

VEGF164, Rat (CHO-expressed)
$1,470.56 Add to cart View Product DetailsVascular Endothelial Growth Factor A164 (VEGF-A164), a member of the cysteine knot growth factor, is one of major isoforms of VEGF-As. VEGF-As are endothelial cell-specific mitogens with angiogenic and vascular permeability-inducing properties. During maturation, rat VEGF-A is alternatively spliced to generate rVEGF-A120, rVEGF-A164 and rVEGF-A188 which correspond to hVEGF-A121, hVEGF-A165 and hVEGF-A189 in human, respectively (the numbers designate the amino acid residues). The active form of rVEGF-A164 is either a homodimeric or heterodimeric polypeptides which bind to the transmembrane tyrosine kinases receptors FLT1, FLK1 or KDR or to the non-tyrosine kinase neuropilin receptors NRP1/2.
-

VEGF165, Human
$1,470.56 Add to cart View Product DetailsVascular Endothelial Growth Factor (VEGF) is a potent growth and angiogenic cytokine. It stimulates proliferation and survival of endothelial cells, and promotes angiogenesis and vascular permeability. Expressed in vascularized tissues, Vascular Endothelial Growth Factor (VEGF) plays a prominent role in normal and pathological angiogenesis. Substantial evidence implicates Vascular Endothelial Growth Factor (VEGF) in the induction of tumor metastasis and intra-ocular neovascular syndromes. Vascular Endothelial Growth Factor (VEGF) signals through the three receptors; fms-like tyrosine kinase (flt-1), KDR gene product (the murine homolog of KDR is the flk-1 gene product) and the flt4 gene product.
-

VEGF165, Human(HEK 293-expressed)
$1,470.56 Add to cart View Product DetailsVascular Endothelial Growth Factor (VEGF) is a potent growth and angiogenic cytokine. It stimulates proliferation and survival of endothelial cells, and promotes angiogenesis and vascular permeability. Expressed in vascularized tissues, Vascular Endothelial Growth Factor (VEGF) plays a prominent role in normal and pathological angiogenesis. Substantial evidence implicates Vascular Endothelial Growth Factor (VEGF) in the induction of tumor metastasis and intra-ocular neovascular syndromes. Vascular Endothelial Growth Factor (VEGF) signals through the three receptors; fms-like tyrosine kinase (flt-1), KDR gene product (the murine homolog of KDR is the flk-1 gene product) and the flt4 gene product.
-
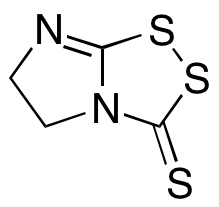
Vegita
$91.43 Add to cart View Product DetailsMolecular Formula : C4 H4 N2 S3
-
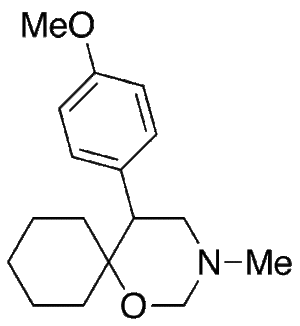
Venlafaxine Cyclic Impurity
$210.45 Add to cart View Product DetailsMolecular Formula : C17 H25 N O2
-

Venlafaxine-d6 N-Oxide
$226.84 Add to cart View Product DetailsMolecular Formula : C17H21D6NO3
-
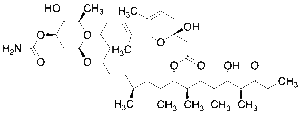
Venturicidin A
$732.26 Add to cart View Product DetailsMolecular Formula : C41H67NO11
-

Veraguensin
$100.91 Add to cart View Product DetailsMolecular Formula : C22H28O5






