50g
Showing 1051–1100 of 1859 results
-
Ethyl Trifluoroacetate
$65.55 Add to cart View Product DetailsMolecular Formula : C4H5F3O2
-
Ethyl-2,2-diethoxyacetate
$188.03 Add to cart View Product DetailsMolecular Formula : C8 H16 O4
-

Ethylene Diamine Tetraacetic Acid Tetrasodium Hydrate
$78.49 Add to cart View Product DetailsMolecular Formula : C10H12N2Na4O8. xH2O
-
Ethylenediamine-N,N,N’,N’-tetraacetic Acid
$221.66 Add to cart View Product DetailsMolecular Formula : C10 H16 N2 O8
-
Ethynylbenzene
$264.79 Add to cart View Product DetailsMolecular Formula : C8 H6
-
Famoxadone
$589.09 Add to cart View Product DetailsMolecular Formula : C22 H18 N2 O4
-

Farnesol (Mixture of Isomers)
$223.39 Add to cart View Product DetailsMolecular Formula : C15 H26 O
-
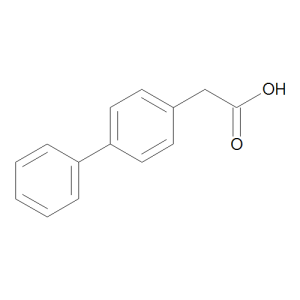
Felbinac
$165.60 Add to cart View Product DetailsMolecular Formula : C14 H12 O2
-

Ferrocene
$94.01 Add to cart View Product DetailsMolecular Formula : 2 C5 H5 . Fe
-

FGF-10, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-10 (FGF-10) is a mitogen mainly produced by mesenchymal stem cells in the lung. FGF-10 belongs to the heparin binding FGF family, and is also known as Keratinocyte Growth Factor-2 (KGF-2). It shares homology with KGF and receptor binding to FGFR2-IIIb. However, while KGF induces proliferation and differentiation of various epithelial cells, FGF-10 promotes budding and branching morphogenesis during the multi-organ development via mesenchymal-epithelial cell interactions. FGF-10 is critical for lung and limb development, and is regulated by Shh during early development.
-

FGF-10, Mouse
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-10 (FGF-10) is a mitogen mainly produced by mesenchymal stem cells in lung. FGF-10 belongs to the heparin binding FGF family, and is also known as Keratinocyte Growth Factor-2 (KGF-2). It shares homology with KGF, and both KGF and FGF-10 activate the receptor FGFR2-IIIb. However, unlike KGF, which induces the proliferation and differentiation of various epithelial cells, FGF-10 is an essential factor for the budding and branching morphogenesis during multi-organ development via mesenchymal-epithelial interactions. FGF-10 is crucial for lung and limb development and is regulated by Shh during early development.
-

FGF-16, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-16 (FGF-16) is a heparin binding growth factor, a member of the FGF family. All FGF family members are heparinbinding growth factors with a core 120 amino acid (aa) FGF domain that allows for a common tertiary structure. FGF family members possess broad mitogenic and cell survival activities, and are involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. The rat homolog is predominantly expressed in embryonic brown adipose tissue and has significant mitogenic activity, which suggests a role in proliferation of embryonic brown adipose tissue. FGF-16 is most similar to FGF-9 (73 % amino acid identity). The protein sequence of human FGF-16 displays 98.6% identity with rat FGF-16. Chimpanzee FGF-16 (207 amino acids), chicken FGF-16 (207 amino acids), and zebrafish FGF-16 (203 amino acids) show 100 %, 89.9 %, and 79.2 % total amino acid identity with human FGF-16.
-
FGF-18, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-18 (FGF-18) is a heparin-binding growth factor that is a member of the FGF family. FGF-18 signals through FGFR 1c, 2c, 3c, and 4. FGF-18 plays an important role in the regulation of cell proliferation, cell differentiation and cell migration. FGF-18 is required for normal ossification and bone development. It can also stimulate hepatic and intestinal proliferation.
-

FGF-18, Rat
$155.25 Add to cart View Product DetailsFibroblast Growth Factor 18 (FGF-18) is a pleiotropic cytokine belonging to the heparin-binding FGF family, which has 23 different members. Structurally, FGF-18 is closely related to FGF-8 and FGF-17. Like other FGFs, FGF-18 can bind to different FGF receptors in vivo. FGF-18 is expressed in various tissues and has multiple functions: during long bone growth, FGF-18 is expressed in perichondrium and developing joints, and regulates bone formation by inhibiting chondrocyte proliferation and differentiation; FGF-18 knock-out mice survive embryonic development, but exhibit skeletal abnormalities and die in the early neonatal period. FGF-18 also induces ectopic cartilage formation in the lung, and alters the morphology of the pulmonary mesenchyma.
-

FGF-21, His, Human
$194.06 Add to cart View Product DetailsFGF-21, also known as fibroblast growth factor-21 and FGFL, is a secreted growth factor belonging to theheparin-binding growth factor family. It is produced by hepatocytes in response to fatty acid stimulation. FGF-21 couples with its co-factor beta-Klotho to signal through FGFR1c and FGFR4. Signal transduction results in insulin-independent uptake of glucose by adipocytes. Clinical administration of FGF-21 induces energy expenditure, fat utilization and lipid excretion.
-
FGF-21, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-21 (FGF-21) is a metabolic cytokine belonging to the heparin-binding FGF family. Along with FGF-19/15 and FGF-23, FGF-21 is categorized as a member of the atypical FGF subfamily, as it must be complexed to the Klotho co-receptor in order to bind to the FGF receptors and activate the downstream signaling pathway. In vivo FGF-21 is expressed in liver, pancreas, adipose tissue, and skeletal muscle, and it plays a central role in the energy metabolism. The expression of FGF-21 is stimulated by free fatty acids and insulin resistant states and is correlated with whole-body insulin resistance. FGF-21 activates glucose uptake in adipocytes and increases insulin sensitivity, implicating it as a novel target with potential anti-diabetic properties.
-

FGF-21, Mouse
$155.25 Add to cart View Product DetailsFibroblast growth factor-21 (FGF21) belongs to the large FGF family which has at least 23 members. Along with FGF-19/15 and FGF-23, FGF-21 is categorized as a member of the atypical FGF subfamily, as it must be complexed to the Klotho co-receptor in order to bind to the FGF receptors and activate the downstream signaling pathway. FGF family members possess broad mitogenic and cell survival activities and are involved in a variety of biological processes including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion.
-

FGF-6, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-6 (FGF-6) is a cytokine belonging to the heparin-binding FGF family, and is structurally related to other members of FGF family, particularly FGF-4. In vivo, FGF-6 exhibits an expression profile predominantly restricted tothe myogenic lineage, and it preferentially binds to two of the FGF receptors: FGFR1 and FGFR4. FGF-6 functions in muscle regeneration, myoblast proliferation and migration, and muscle differentiation in a dose-dependent manner. In vivo high concentration of recombinant FGF-6 up-regulates and down-regulates FGFR1 and FGFR4, respectively, as FGFR1 promotes the proliferation while FGFR4 promotes the differentiation in the muscle. Besides its dual function in muscle regeneration, FGF-6 may act as a regulator of bone metabolism as well.
-

FGF-8, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor-8 (FGF-8) is a heparin-binding growth factor of the FGF family. There are 4 known forms of FGF8 produced by alternative splicing: FGF8a, FGF-8b, FGF-8e and FGF-8f. The human and mouse FGF8b are identical of aa sequences. FGF-8 plays an important role in the regulation of embryonic development, cell proliferation, cell differentiation and cell migration. FGF-8 is required for normal brain, eye, ear and limb development during embryogenesis. It is also required for normal development of the gonadotropin- releasing hormone (GnRH) neuronal system.
-

FGF-8a, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor 8a (FGF-8a) is a cytokine belonging to the heparin-binding FGF family, which has at least 23 members. FGF-8 has 8 different isoforms, named FGF-8a through FGF-8h. Different FGF-8 isoforms have different affinities to the receptors, and thus participate in different signaling cascade pathways. FGF-8 has very widespread expression during embryonic development, and is an organizer and inducer for gastrulation, somitogenesis, morphogenesis, and limb induction. However, FGF-8 is also a potential oncogene: in normal adult cells, FGF-8 has very low expression, but FGF-8 is highly expressed in cancer cells of breast, prostate, and ovarian tumors. FGF-8 promotes tumor angiogenesis by increasing neovascularization, and induces osteoblastic differentiation.
-

FGF-8e, Human
$155.25 Add to cart View Product DetailsFibroblast Growth Factor 8e (FGF-8e) is a cytokine belonging to the heparin-binding FGF family, which has at least 23 members. FGF-8 has 8 different isoforms, named FGF-8a through FGF-8h. Different FGF-8 isoforms have different receptor affinities, and thus participate in different signaling cascade pathways. FGF-8 has widespread expression during embryonic development, promoting gastrulation, somitogenesis, morphogenesis, and limb formation. FGF-8 also has oncogenic potential. While in normal cells FGF-8 is expressed at very low levels, in breast, prostate and ovarian cancer FGF-8 is highly expressed.FGF-8 promotes tumor angiogenesis by increasing neovascularization, and inducing osteoblastic differentiation.
-

FGF-9, Human
$194.06 Add to cart View Product DetailsFibroblast Growth Factor-9 (FGF-9) is a heparin binding growth factor that belongs to the fibroblast growth factor (FGF) family. FGF family members possess broad mitogenic and cell survival activities, and are involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. FGF-9 was isolated as a secreted factor that exhibits a growth-stimulating effect on cultured glial cells. In nervous system, this protein is produced mainly by neurons and may be important for glial cell development.
-
FGF-acidic, Human
$86.25 Add to cart View Product DetailsFibroblast Growth Factor- acidic (FGF-acidic), also known as FGF-1 and endothelial cell growth factor, is a member of the FGF family which currently contain 23 members. FGF acidic and basic, unlike the other members of the family, lack signal peptides and are apparently secreted by mechanisms other than the classical protein secretion pathway. FGF acidic has been detected in large amounts in the brain. Other cells known to express FGF acidic include hepatocytes, vascular smooth muscle cells, CNS neurons, skeletal muscle cells, fibroblasts, keratinocytes, endothelial cells, intestinal columnar epithelium cells and pituitary basophils and acidophils. As with other FGF’s, FGF acidic exhibits considerable species cross reactivity. FGF acidic and FGF basic stimulate the proliferation of all cells of mesodermal origin, and many cells of neuroectodermal, ectodermal and endodermal origin.
-

FGF-acidic, Mouse
$86.25 Add to cart View Product DetailsFibroblast Growth Factor- acidic (FGF-acidic) is a mitogen targeting at the endothelial cells, and belongs to the heparin binding FGF family, which contains 22 members. FGF-acidic binds to the receptor family FGFR1-4 in vivo with the assistance of heparin. However, along with FGF -basic, FGF-acidic lacks the signal peptide segment, and thus is not secreted via endoplasmic reticulum (ER) and Golgi bodies. Studies have shown that FGF-acidic is highly regulated, and it is a direct angiogenesis factor. If unregulated, angiogenesis could contribute to several diseases including arthritis, diabetes, ocular neovascularization, and especially tumors. Therefore, FGF-acidic is treated as a potential oncogene, and its overexpression is correlated tightly with several cancers.
-

FGF-basic (145aa), Human
$90.56 Add to cart View Product DetailsFibroblast Growth Factor-basic (FGF-basic), also known as FGF-2, is a pleiotropic cytokine and one of the prototypic members of the heparin-binding FGF family. Like other FGF family members, FGF-basic has the β trefoil structure. In vivo, FGF-basic is produced by a variety of cells, including cardiomycotes, fibroblasts, and vascular cells. FGF-basic regulates a variety of processes including cell proliferation, differentiation, survival, adhesion, motility, apoptosis, limb formation and wound healing. FGF-basic can be tumorigenic due to its role in angiogenesis and blood vessel remodeling. The angiogenic effects of FGF-basic can produce beneficial cardioprotection during acute heart injury.
-

FGF-basic (146aa), Human
$86.25 Add to cart View Product DetailsFibroblast Growth Factor-basic (FGF-basic), also known as FGF-2, is a pleiotropic cytokine and one of the prototypic members of the heparin-binding FGF family. Like other FGF family members, FGF-basic has the β trefoil structure. In vivo, FGF-basic is produced by a variety of cells, including cardiomycotes, fibroblasts, and vascular cells. FGF-basic regulates a variety of processes including cell proliferation, differentiation, survival, adhesion, motility, apoptosis, limb formation and wound healing. FGF-basic can be tumorigenic due to its role in angiogenesis and blood vessel remodeling. The angiogenic effects of FGF-basic can produce beneficial cardioprotection during acute heart injury.
-

FGF-basic (154aa), Human
$86.25 Add to cart View Product DetailsFibroblast Growth Factor-basic (FGF-basic), also known as FGF-2, is a pleiotropic cytokine and one of the prototypic members of the heparin-binding FGF family. Like other FGF family members, bFGF has the β trefoil structure. In vivo, bFGF is produced by a variety of cells, including cardiomycotes, fibroblasts, and vascular cells. bFGF regulates a variety of processes including cell proliferation, differentiation, survival, adhesion, motility, apoptosis, limb formation and wound healing. bFGF can be tumorigenic due to its role in angiogenesis and blood vessel remodeling. The angiogenic effects of bFGF can produce beneficial cardioprotection during acute heart injury.
-

FGF-basic, Bovine
$86.25 Add to cart View Product DetailsFibroblast Growth Factor-basic (FGF-basic), also known as FGF-2, is a pleiotropic cytokine and one of the prototypic members of the heparin-binding FGF family. Like other FGF family members, FGF-basic has the β trefoil structure. In vivo, FGF-basic is produced by a variety of cells, including cardiomycotes, fibroblasts, and vascular cells. FGF-basic regulates a variety of processes including cell proliferation, differentiation, survival, adhesion, motility, apoptosis, limb formation and wound healing. FGF-basic can be tumorigenic due to its role in angiogenesis and blood vessel remodeling. The angiogenic effects of FGF-basic can produce beneficial cardioprotection during acute heart injury.
-
FGF-basic, Mouse
$86.25 Add to cart View Product DetailsFibroblast Growth Factor-basic (FGF-basic), also known as HBGF-2, is a non-glycosylated heparin-binding growth factor that belongs to the FGF family. FGF-basic is present in basement membranes and in the subendothelial extracellular matrix of blood vessels. FGF-basic signals through FGFR1, 2, 3 and 4 that plays an important role in the regulation of cell survival, cell division, angiogenesis, cell differentiation and cell migration.
-
FGF-basic, Rat
$86.25 Add to cart View Product DetailsFibroblast Growth Factor-basic (FGF-basic), also known as FGF-2, is a pleiotropic cytokine and one of the prototypic members of the heparin-binding FGF family. Like other FGF family members, FGF-basic has the β trefoil structure. In vivo, FGF-basic is produced by a variety of cells, including cardiomycotes, fibroblasts, and vascular cells. FGF-basic regulates a variety of processes including cell proliferation, differentiation, survival, adhesion, motility, apoptosis, limb formation and wound healing. FGF-basic can be tumorigenic due to its role in angiogenesis and blood vessel remodeling. The angiogenic effects of FGF-basic can produce beneficial cardioprotection during acute heart injury.
-

FGF-R2β Fc Chimera, Mouse
$137.14 Add to cart View Product DetailsFibroblast growth factor receptor 2 (FGFR2) also known as CD332 is a receptor for fibroblast growth factor and it has important roles in embryonic development and tissue repair, especially bone and blood vessels. FGFR2 has two naturally occurring isoforms, FGFR2IIIb and FGFR2IIIc, created by splicing of the third immunoglobulin-like domain. FGFR2IIIb is predominantly found in ectoderm derived tissues and endothelial organ lining. Like the other members of the fibroblast growth factor receptor family, these receptors signal by binding to their ligand and dimerisation (pairing of receptors), which causes the tyrosine kinase domains to initiate a cascade of intracellular signals. On a molecular level these signals mediate cell division, growth and differentiation.
-

FGF-R3(ⅢC) Fc Chimera, Mouse
$137.14 Add to cart View Product DetailsFibroblast growth factor receptor 3(FGFR3) also known as CD333 (cluster of differentiation 333) is a member of the fibroblast growth factor receptor family, where amino acid sequence is highly conserved between members and throughout evolution. The FGFR3 gene produces various forms of the FGFR3 protein and the location varies depending on the isoform of the FGFR3 protein. Since the different forms are found within different tissues, the protein is responsible for multiple growth factor interactions. Gain of function mutations in FGFR3 inhibits chondrocyte proliferation and underlies achondroplasia and hypochondroplasia.
-

FGL1 Fc Chimera, Human
$137.14 Add to cart View Product DetailsFGL1 (Fibrinogen-like protein 1), also known as hepatocyte-derived fibrinogen-related protein 1 (HFREP-1), HP-041, hepassocin (HPS), and liver fibrinogen-related protein 1 (LFIRE-1), is a liver-specific secreted protein belonging to the fibrinogen superfamily whose members share a fibrinogen domain at their C-termini. FGL1 is an immune suppressive molecule that inhibits the activation of antigen-specific T cells by acting as a major ligand of LAG3, and binds LAG3 independently of MHC class II.
-

Flt-3L, His, Mouse
$224.25 Add to cart View Product DetailsFlt3L, also known as Fms-related tyrosine kinase 3 ligand and SL cytokine, is a single-pass type I membrane protein. It is expressed by stromal cells and T cells. Flt3L signals through tyrosine kinase receptor Flt3/Flk2 to stimulate the proliferation of early hematopoietic progenitor cells. It synergizes with other growth factors, such as GM-CSF, IL-3 and CSF, to promote the differentiation of both myeloid and lymphoid cells. Alternative splicing and proteolytic cleavage of membrane-bound Flt3L generates a soluble extracellular domain (ECD) isoform with full biological activity.
-

Flt-3L, Human
$224.25 Add to cart View Product DetailsFms-related tyrosine kinase 3 ligand (Flt3L) is growth fator stimulates the proliferation and differentiation of hematopoietic multipotent progenitors and promotes proliferation of NK cells and dendritic cell subgroups by combination with other growth factors. Flt3L is produced by T cells and stromal fibroblasts, and targeted various cells including hematopoietic stem cells, B cells, T cells, dendritic cells, and NK cells. Flt3L binds to it cognate tyrosine kinase receptor Flt3 and activates JAK/STAT signaling pathway.Flt3L is a hematopoietic four helical bundle cytokine with structurally homologous to stem cell factor (SCF) and colony stimulating facor 1 (CSF-1) demonstrated four conserved cysteines and two glycosylation sites. Flt3L naturally as a non-disulfide-linked homodimer with multiple isoforms. The extracellular portion is approximately 160 amino acid residues in length and the cytoplasmic segment is approximately 20-30 amino acid residues in length.
-
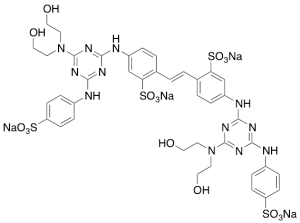
Fluorescent Brightener 87 (Technical Grade)
$233.74 Add to cart View Product DetailsMolecular Formula : C40H40N12Na4O16S4
-
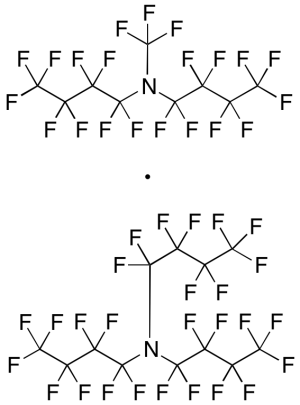
Fluorinert FC 40 (Technical Grade)
$130.24 Add to cart View Product DetailsMolecular Formula : C9F21N •C12F27N
-
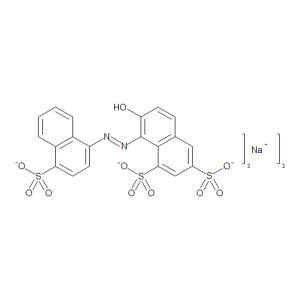
Food Red No. 102 (>80%)
$378.64 Add to cart View Product DetailsMolecular Formula : C20 H11 N2 O10 S3 . 3 Na
-
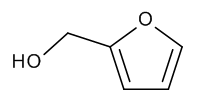
Furfuryl Alcohol
$82.80 Add to cart View Product DetailsMolecular Formula : C5 H6 O2
-

G-CSF, Human
$245.81 Add to cart View Product DetailsGranulocyte Colony-Stimulating Factor (G-CSF) contains internal disulfide bonds. Among the family of colony-stimulating factors, Granulocyte Colony Stimulating Factor (G-CSF) is the most potent inducer of terminal differentiation to granulocytes and macrophages of leukemic myeloid cell lines. The synthesis of Granulocyte Colony Stimulating Factor (G-CSF) can be induced by bacterial endotoxins, TNF, Interleukin-1 and GM-CSF. Prostaglandin E2 inhibits the synthesis of Granulocyte Colony Stimulating Factor (G-CSF). In epithelial, endothelial, and fibroblastic cells secretion of Granulocyte Colony Stimulating Factor (G-CSF) is induced by Interleukin-17.
-

G-CSF, Human(CHO-expressed)
$271.69 Add to cart View Product DetailsHuman Granulocyte Colony Stimulating Factor (G-CSF) contains internal disulfide bonds. Among the family of colony-stimulating factors, Granulocyte Colony Stimulating Factor (G-CSF) is the most potent inducer of terminal differentiation to granulocytes and macrophages of leukemic myeloid cell lines. The synthesis of Granulocyte Colony Stimulating Factor (G-CSF) can be induced by bacterial endotoxins, TNF, Interleukin-1 and GM-CSF. Prostaglandin E2 inhibits the synthesis of Granulocyte Colony Stimulating Factor (G-CSF). In epithelial, endothelial, and fibroblastic cells, the secretion of Granulocyte Colony Stimulating Factor (G-CSF) is induced by Interleukin-17.
-

G-CSF, Mouse
$271.69 Add to cart View Product DetailsGranulocyte Colony-Stimulating Factor (G-CSF), also known as CSF-3 and MGI-1G, is a cytokine and hormone belonging to the IL-6 superfamily. It is expressed by monocytes, macrophages, endothelial cells, fibroblasts and bone marrow stroma. G-CSF stimulates the bone marrow to produce granulocytes and stem cells, and specifically stimulates the proliferation and differentiation of the neutrophilic granulocyte lineage. G-CSF has been used to stimulate white blood cell production after chemotherapy. It has also been used to boost the number of hematopoietic stem cells after bone marrow transplantation.
-

gAcrp30/Adipolean, Mouse
$155.25 Add to cart View Product DetailsgAcrp30 is the globular head domain of Adipocyte complement-related protein of 30 kDa (Acrp30), a cytokine expressed in adipocytes. The name of Acrp30 is bases on its closest homolog, complement factor c1q, and the globular domain of Acrp30 has an unexpected homology with the Tumor Necrosis Factor (TNF) family of cytokines. Acrp30 is recognized by two receptors: adipoR1 expressed in skeletal muscle, and adipoR2 expressed in liver. The expression level of Acrp30 in adipocytes is negatively correlated with body weight and is lower in obese mouse than normal mouse. The globular domain of Acrp30 induces free fatty acid oxidation in muscle and weight reduction in mouse, suggesting its potential use as a pharmacological agent in obesity.
-
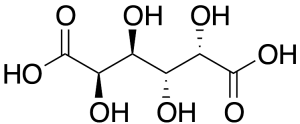
Galactaric Acid
$56.06 Add to cart View Product DetailsMolecular Formula : C6 H10 O8
-
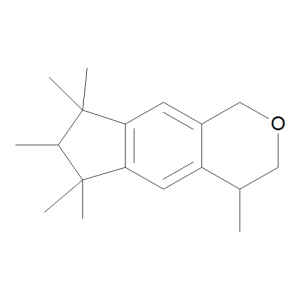
Galaxolide (solution 50% in diethyl phthalate)
$87.98 Add to cart View Product DetailsMolecular Formula : C18 H26 O
-
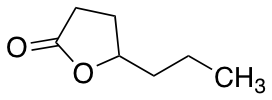
gamma-Heptalactone
$65.55 Add to cart View Product DetailsMolecular Formula : C7 H12 O2
-

Gamma-Oryzanol
$77.63 Add to cart View Product DetailsMolecular Formula : C40 H58 O4
-
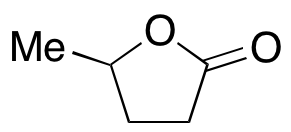
gamma-Valerolactone
$225.98 Add to cart View Product DetailsMolecular Formula : C5 H8 O2
-

Garlic Extract (Contains Diallyl Trisulfide, Technical Grade)
$82.80 Add to cart View Product DetailsMolecular Formula : No Data Available
-
GDNF, Human
$271.69 Add to cart View Product DetailsGlial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor belonging to the TGF-beta super family and is necessary for neuron survival and phenotypic maintenance in the central and peripheral nervous systems. G-DNF has the potential to support the differentiation and survival of many neuron subpopulations, especially dopaminergic neurons and motor neurons, as well as Purkinje cells and sympathetic neurons. Sertoli cells, type 1 astrocytes, Schwann cells, neurons, pinealocytes and skeletal muscle cells are known to express GDNF in human. GDNF has been shown to interact with GFRA2 and GDNF family receptor alpha 1. Mutations in this gene may be associated with Hirschsprung’s disease, Parkinson’s disease and amyotrophic lateral sclerosis (ALS).The recombinant human G-DNF expressed in E.coli is a disulfide-linked homo-dimer, with an apparent molecular weight of 17 kDa.






