50g
Showing 1151–1200 of 1859 results
-
ICOS Fc Chimera, Human
$189.75 Add to cart View Product DetailsInducible T-cell costimulator is an immune checkpoint protein that in humans is encoded by the ICOS gene. CD278 or ICOS (Inducible T-cell COStimulator) is a CD28-superfamily costimulatory molecule that is expressed on activated T cells. It is thought to be important for Th2 cells in particular. The protein encoded by this gene belongs to the CD28 and CTLA-4 cell-surface receptor family. It forms homodimers and plays an important role in cell-cell signaling, immune responses and regulation of cell proliferation.
-

IFN-α 2a, Human
$68.14 Add to cart View Product DetailsInterferon-Alpha 2a (IFN-Alpha 2a), Human produced by leukocytes is a member of Interferon family. IFN-alpha is mainly involved in innate immune response against a broad range of viral infections. IFN-alpha 2 has three acid stable forms (a,b,c) signaling through IFNAR2. IFN-alpha 2a shares 99.4% , 98.8% aa sequence identity with IFN-alpha 2b and 2c respectively. IFN-alpha contains four highly conserved cysteine residues which form two disulfide bonds, one of which is necessary for biological activity.
-

IFN-α 2b, Human
$86.25 Add to cart View Product DetailsInterferon-Alpha 2b (IFN-Alpha 2b) produced by leukocytes is a member of Interferon family. IFN-alpha is mainly involved in innate immune response against a broad range of viral infections. IFN-alpha 2 has three acid stable forms (a,b,c) signaling through IFNAR2. IFN-alpha 2b shares 99.4% aa sequence identity with both IFN-alpha 2a and 2c. IFN-alpha contains four highly conserved cysteine residues which form two disulfide bonds, one of which is necessary for biological activity.
-

IFN-γ R II, Human
$133.69 Add to cart View Product DetailsIFN-gamma Receptor II, also known as IFNGR2 and IFNGT1, is a transmembrane protein belonging to the type II cytokine receptor family. IFNGR2 is a non-ligand-binding beta chain of the IFN-gamma receptor. It is an integral part of the IFN-gamma signaling transduction pathway and is likely to interact with GAF, JAK1 and JAK2. Defects in IFNGR2 are a cause of autosomal recessive Mendelian susceptibility to mycobacterial disease (MSMD), also known as familial disseminated atypical mycobacterial infection.
-

IFN-γ, Human
$72.45 Add to cart View Product DetailsHuman Interferon gamma (hIFN-γ) is amacrophage‐activating factor and the lone member of Interferon type II.The active form of IFN-γ is an antiparallel dimer that interacts with the receptor IFN-γR1 and sets off IFN-γ/JAK/STAT pathway. IFN-γ signaling does diverse biological functions primarily related to host defense and immune regulation, including antiviral and antibacterial defense, apoptosis, inflammation, and innate and acquired immunity. While IFN-γ–induced inflammatory cascade summons a variety of immune‐related cell types, such as macrophages, natural killer (NK) cells and cytotoxic T lymphocytes (CTLs), IFN-γ is also implicated in resistance to NK cell and CTL responses and in immune escape in a variety of cancers.
-

IFN-γ, Human(CHO-expressed)
$76.76 Add to cart View Product DetailsHuman Interferon gamma (hIFN-γ) is amacrophage-activating factor and the lone member of Interferon type II. The active form of IFN-γ is an antiparallel dimer that interacts with the receptor IFN-γR1 and sets off IFN-γ/JAK/STAT pathway. IFN-γ signaling does diverse biological functions primarily related to host defense and immune regulation, including antiviral and antibacterial defense, apoptosis, inflammation, and innate and acquired immunity. While IFN-γ–induced inflammatory cascade summons a variety of immune-related cell types, such as macrophages, natural killer (NK) cells and cytotoxic T lymphocytes (CTLs), IFN-γ is also implicated in resistance to NK cell and CTL responses and in immune escape in a variety of cancers.
-

IFN-γ, Rat (CHO-expressed)
$76.76 Add to cart View Product DetailsInterferon-γ (IFN-γ), also known as Type II interferon or immune interferon, is a cytokine produced primarily by T-lymphocytes and natural killer cells. The active form of IFN-γ is an antiparallel dimer that interacts with the receptor IFN-γR1 and sets off IFN-γ/JAK/STAT pathway. IFN-γ signaling does diverse biological functions primarily related to host defense and immune regulation, including antiviral and antibacterial defense, apoptosis, inflammation, and innate and acquired immunity. While IFN-γ–induced inflammatory cascade summons a variety of immune-related cell types, such as macrophages, natural killer (NK) cells and cytotoxic T lymphocytes (CTLs), IFN-γ is also implicated in resistance to NK cell and CTL responses and in immune escape in a variety of cancers.
-
IGEPAL CA-630
$89.70 Add to cart View Product DetailsMolecular Formula : (C2H4O)nC14H22O
-
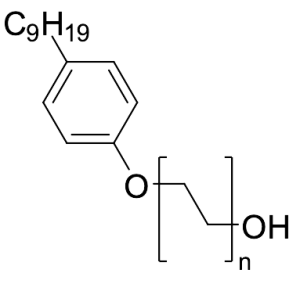
Igepal CO-520
$106.09 Add to cart View Product DetailsMolecular Formula : (C2H4O)n.C15H24O
-

IGF-BP-2, His, Human
$224.25 Add to cart View Product DetailsIGF-BP-2, also known as Insulin-like growth factor-binding protein 2, IBP-2 and BP-2, is a cysteine-rich secreted protein belonging to the IGF-binding protein superfamily. It is expressed by the central nervous system, bone cells and reproductive tissues. IGF-BP-2 binds to both IGF-I and IGF-II, with a much higher binding affinity to IGF-II than IGF-I. IGF-BP-2 has been shown to inhibitand stimulate the growth promoting effects of IGFs, thus serving as a regulator for IGF distribution, function and activity.
-

IGF-BP-4, His, Human
$224.25 Add to cart View Product DetailsInsulin-like growth factor-binding protein 4 (IGF-BP-4), also known as IBP-4, is a secreted glycoprotein belonging to the IGFBP family. IGF-BP-4 is produced by osteoblasts, epidermis, ovarian follicles and other tissues. It binds both insulin-like growth factor (IGF) I and II, and it circulates in the plasma in both glycosylated and non-glycosylated forms. IGF-BP-4 prolongs the half-life of the IGFs and has been shown to inhibit or stimulate the growth-promoting effects of the IGFs. Pregnancy Associated Plasma Protein A (PAPP-A) proteolytically cleaves IGF-BP-4 and reduces its affinity to bind IGFs, and thus serves as an important regulator of IGF-BP-4 function.
-

IGF-I, Bovine
$76.76 Add to cart View Product DetailsInsulin-like growth factor 1 (IGF-1), also called Somatomedin, is a hormone similar in molecular structure to insulin but has a much higher growth-promoting activity. IGF-1 consists of 70 amino acids in a single chain with three intramolecular disulfide bridges. IGF-1 may be a physiological regulator of [1-14C]-2-deoxy-D-glucose (2DG) transport and glycogen synthesis in osteoblasts. It is able to stimulate glucose transport in bone-derived osteoblastic (PyMS) cells and is effective at much lower concentrations than insulin, not only regarding glycogen and DNA synthesis but also with regard to enhancing glucose uptake. It may also play a role in synapse maturation.
-
IGF-I, Human
$76.76 Add to cart View Product DetailsInsulin-like growth factor I (IGF-I) also known as Somatamedin C is a hormone similar in molecular structure to insulin. Human IGF-I has two isoforms (IGF-IA and IGF-IB) which are differentially expressed by various tissues. Mature human IGF-I shares 94% and 96% aa sequence identity with mouse and rat IGF-I, respectively. Both IGF-I and IGF-II (another ligand of IGF) can signal through the IGF-I receptor (IGFIR), but only IGF-II can bind the IGF-II receptor (IGFIIR/ Mannose-6-phosphate receptor). IGF-I plays an important role in childhood growth and continues to have anabolic effects in adults.
-

IGF-I, Mouse
$86.25 Add to cart View Product DetailsInsulin-like Growth Factor I (IGF-I) is a single chain 7 kDa growth-promoting polypeptide originally identified as somatomedin-c. It belongs to the IGF family of peptides, which also includes IGF-II and insulin. The gene expression of IGF-I is mainly regulated by Growth Hormone, and IGF-I executes its functions via signaling through transmembrane tyrosine receptors (IGF Receptors). Most circulating IFG-I is associated with the IGF Binding Protein 3 (IGFBP-3), and the IGFBPs may inhibit the actions of IGFs by competing against the IGF Receptors. IGF-I is active in post-natal and adult animals, and is crucial for somatic growth, as IGF-I null mice show marked retardation in utero. IGF-I is involved in carcinogenesis, and related to prostate cancer as well.
-

IGF-I, Rat
$76.76 Add to cart View Product DetailsInsulin-like Growth Factor I (IGF-I) is a single chain 7 kDa growth-promoting polypeptide originally identified as somatomedin-c. It belongs to the IGF family of peptides, which also includes IGF-II and insulin. The gene expression of IGF-I is mainly regulated by Growth Hormone, and IGF-I executes its functions via signaling through transmembrane tyrosine receptors (IGF Receptors). Most circulating IFG-I is associated with the IGF Binding Protein 3 (IGFBP-3), and the IGFBPs may inhibit the actions of IGFs by competing against the IGF Receptors. IGF-I is active in post-natal and adult animals, and is crucial for somatic growth, as IGF-I null mice show marked retardation in utero. IGF-I is involved in the carcinogenesis, and related to the prostate cancer as well.
-

IGF-II, Human
$86.25 Add to cart View Product DetailsInsulin-like Growth Factor II (IGF-II) is a single chain 7 kDa polypeptide, and shares a high degree of homology with insulin. During circulation in vivo, IGF-II is complexed to high affinity binding proteins, IGF Binding Proteins (IGFBP), which act as circulating reservoirs, transport IGF-II, and prolong the half life of IGF-II. The receptors of IGF-II (IGFRs) are transmembrane tyrosine receptors, and are heterotetrameric consisting of two α-subunits and two β-subunits. IGFRs execute their role via intracellullar signaling molecules, such as IRS, shc, and PI3K. The functions of IGF-II include promoting cell survival, growth, proliferation, differentiation and motility. In particular, IGF-II promotes proliferation, inhibits death, and stimulates transformation in breast cancer cells.
-
IHH, Human
$155.25 Add to cart View Product DetailsThe Indian Hedgehog protein (IHH) is one of three proteins in the mammalian hedgehog family, the others being desert hedgehog (DHH) and Sonic hedgehog (SHH). Hedgehog proteins are important signaling molecules during embryonic development and are highly conserved across species. Mouse and human IHH share 100% amino acid identity in the signaling domain, while mouse IHH and SHH share 90% amino acid identity in the N-terminal signaling domain. IHH mRNA expression is detected in fetal lung, gut, stomach, liver, kidney, pancreas and strongly in cartilage in growth regions of the developing bone. IHH has a specific role in bone growth and differentiation. In addition, IHH is involved in yolk sac vasculogenesis, having a central role in differentiation of epiblast cells into endothelial and red blood cells. IHH gene mutations cause the brachydactyly type A1 which is characterized by shortening or malformation of the phalanges and also the acrocapitofemoral dysplasia.
-

IL-1 RA, Human(HEK 293-expressed)
$76.76 Add to cart View Product DetailsIL-1 Receptor Antagonist, also known as IL-1RA, ICIL-1RA, IRAP and IL-1RN, is a member of the interleukin 1 cytokine family. It is expressed by monocytes, neutrophils, macrophages, epithelial cells and fibroblasts. IL-1RA inhibits the activity of both IL-1alpha and IL-1beta, and modulates a variety of IL-1 related immune and inflammatory responses. It inhibits the activity of IL-1 by binding to the receptor IL-1R1 and preventing its association with the coreceptor IL-1RAP for signaling. Clinical studies are being conducted to investigate the use of IL-1RA in the treatment of sepsis, rheumatoid arthritis and chronic myelogenous leukemia.
-

IL-10, Human(CHO-expressed)
$271.69 Add to cart View Product DetailsInterleukin-10 (IL-10), initially known as Cytokine Synthesis Inhibitory Factor (CSIF), belongs to the IL-10 family and shares more than 80% sequence homology with Epstein-Barr Virus protein BCRFI. It is produced by many immune cells, such as T-cells, macrophages, mast cells, and dendritic cells. It is usually secreted as a homodimer and, upon binding to its receptor, inhibits the synthesis of a number of cytokines, including IFN-gamma, IL-2, IL-3, TNF and GM-CSF produced by activated macrophages and Th2 cells. It also displays ability to suppress Antigen-Presenting Cell (APC) function. The net effect of Interleukin-10 appears to be inhibitory; however, stimulatory effects, such as stimulation of B cell maturation and antibody production, are also reported.
-

IL-10, Mouse(CHO-expressed)
$271.69 Add to cart View Product DetailsInterleukin-10 (IL-10), initially known as Cytokine Synthesis Inhibitory Factor (CSIF), belongs to the IL-10 family and shares more than 80% sequence homology with the Epstein-Barr Virus protein BCRFI. It is produced by many immune cells, such as T-cells, macrophages, mast cells and dendritic cells. It is usually secreted as a homodimer and, upon binding to its receptor, inhibits the synthesis of a number of cytokines, including IFN-gamma, IL-2, IL-3, TNF and GM-CSF, by activated macrophages and Th2 cells. It also displays the ability to suppress Antigen-Presenting Cell (APC) function. The net effect of Interleukin-10 appears to be inhibitory; however, stimulatory effects, such as stimulation of B cell maturation and antibody production, are also reported.
-

IL-10, Rat (CHO-expressed)
$271.69 Add to cart View Product DetailsInterleukin-10 (IL-10), initially known as Cytokine Synthesis Inhibitory Factor (CSIF), belongs to the IL-10 family and shares more than 80% sequence homology with the Epstein-Barr Virus protein BCRFI. It is produced by many immune cells, such as T-cells, macrophages, mast cells and dendritic cells. It is usually secreted as a homodimer and, upon binding to its receptor, inhibits the synthesis of a number of cytokines, including IFN-gamma, IL-2, IL-3, TNF and GM-CSF produced by activated macrophages and Th2 cells. It also displays the ability to suppress Antigen-Presenting Cell (APC) function. The net effect of Interleukin-10 appears to be inhibitory; however, stimulatory effects, such as stimulation of B cell maturation and antibody production, are also reported.
-

IL-11 Fc Chimera, Human
$319.13 Add to cart View Product DetailsInterleukin-11 is a pleiotropic cytokine that was originally detected in the conditioned medium ofan IL-1α-stimulated primate bone marrow stromal cell line (PU-34) as a mitogen for the IL-6-responsive mouse plasmacytoma cell line T11. IL-11 has multiple effects on both hematopoietic and non-hematopoietic cells. Many of the biological effects described for IL-11 overlap with those for IL-6. In vitro, IL-11 can synergize with IL-3, IL-4 and SCF to shorten the G0 period of early hematopoietic progenitors. IL-11 alsoenhances the IL-3-dependent megakaryocyte colony formation. IL-11 has been found to stimulate the T cell-dependent development of specific immunoglobulin-secreting B cells.
-

IL-11, Mouse(HEK 293-expressed)
$271.69 Add to cart View Product DetailsInterleukin-11 (IL-11) is a pleiotropic cytokine that was originally detected in the conditioned medium of an IL-1α-stimulated primate bone marrow stromal cell line (PU-34) as a mitogen for the IL-6-responsive mouse plasmacytoma cell line T11. IL-11 contains no cysteine residues or potential glycosylation sites. IL-11 has multiple effects on both hematopoietic and nonhematopoietic cells. Many of the biological effects described for IL-11 overlap those for IL-6. In vitro, IL-11 can synergize with IL-3, IL-4 and SCF to shorten the G0 period of early hematopoietic progenitors. IL-11 also enhances the IL-3-dependent megakaryocyte colony formation. IL-11 has been found to stimulate the T cell dependent development of specific immunoglobulin-secreting B cell.
-

IL-12, His, Rat
$306.19 Add to cart View Product DetailsInterleukin-12 (IL-12), also known as NKSF, TCMF, CLMF and TSF, is a heterodimeric cytokine composed of p35 and p40 subunits. It is produced by monocytes, macrophages, B cells and dendritic cells in response to bacterial lipopolysaccharides and intracellular pathogens. IL-12 signals through the IL-12 receptor complex, which is comprised of IL-12 Rβ1 and IL-12 Rβ2. IL-12 induces the proliferation and activation of hematopoietic stem cells, natural killer cells and T- cells. It is indispensible during the development of Th1 cells, leading to the production of IFN-gamma and IL-2.
-

IL-12, Mouse
$439.88 Add to cart View Product DetailsInterleukin-12 (IL-12), also known as NKSF, TCMF, CLMF and TSF, is a heterodimeric cytokine composed of p35 and p40 subunits. It is produced by monocytes, macrophages, B cells and dendritic cells in response to bacterial lipopolysaccharides and intracellular pathogens. IL-12 signals throughtheIL-12 receptor complex, which is comprised of IL-12 Rβ1 and IL-12 Rβ2. IL-12 induces the proliferation and activation of hematopoietic stem cells, natural killer cells and T- cells. It is indispensible during the development of Th1 cells, leading to the production of IFN-gamma and IL-2.
-

IL-13, His, Mouse(CHO-expressed)
$271.69 Add to cart View Product DetailsInterleukin-13 (IL-13), also known as T-cell activation protein P600, is an immunoregulatory cytokine belonging to the IL-4/IL-13 family. It is produced by activated Th2 cells, mast cells and NK cells. IL-13 signals through a receptor complex composed of IL-4Rα and IL13Rα1 (or IL13Rα2). IL-13 inhibits the expression of inflammatory cytokines such as IL-1β, TNF-α and IL-6 by monocytes and macrophages. It also induces B cell activation and IgE secretion.
-

IL-13, Human(CHO-expressed)
$271.69 Add to cart View Product DetailsInterleukin 13 (IL-13) is an immunoregulatory cytokine produced primarily by activated Th2 cells, and also by mast cells and NK cells. Targeted deletion of IL-13 in mice resulted in impaired Th2 cell development and indicated an important role for IL-13 in the expulsion of gastrointestinal parasites. IL-13 exerts anti-inflammatory effects on monocytes and macrophages and it inhibits the expression of inflammatory cytokines such as IL-1beta, TNF-alpha, IL-6 and IL-8. IL-13 has also been shown to enhance B cell proliferation and to induce isotype switching resulting in increased production of IgE. Blocking of IL-13 activity inhibits the pathophysiology of asthma. Human and mouse IL-13 is cross-species reactive.
-

IL-15, His, Human
$207.00 Add to cart View Product DetailsInterleukin-15 (IL-15) is a cytokine with structural similarity to IL-2. Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain (gamma-C, CD132). IL-15 is secreted by mononuclear phagocytes (among other cells) following infection by virus. This cytokine induces cell proliferation of natural killer cells, which are cells of the innate immune system whose principal role is to kill virally infected cells. IL-15 can stimulate the proliferation of T-lymphocytes. Stimulation by IL-15 occurs following its interaction with IL-15Rα. This interaction may enhance IL-15’s interaction with IL15Rβγc.
-

IL-15, Human
$207.00 Add to cart View Product DetailsInterleukin-15 (IL-15) is a cytokine with structural similarity to IL-2. Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain (gamma-C, CD132). IL-15 is secreted by mononuclear phagocytes (among other cells) following infection by virus. This cytokine induces cell proliferation of natural killer cells, which are cells of the innate immune system whose principal role is to kill virally infected cells. IL-15 can stimulate the proliferation of T-lymphocytes. Stimulation by IL-15 occurs following its interaction with IL-15Rα. This interaction may enhance IL-15’sinteraction with IL15Rβγc.
-

IL-16, Human
$465.75 Add to cart View Product DetailsIL-16 is a CD8+ T cell-derived cytokine that induces chemotaxis of CD4+ T cells and CD4+ monocytes and eosinophils. Analysis by gel filtration suggests that, under physiological conditions, human IL-16 exists predominantly as a noncovalently linked multimer, but that some IL-16 may exist as a monomer. However, only the multimeric form appears to possess chemotactic activity, suggesting that receptor cross-linking may be required for activity. IL-16 also induces expression of IL-2 receptor (IL-2R) and MHC class II molecules on CD4+ T cells. Human and murine IL-16 show significant cross-species reactivity.
-

IL-17A, His, Human
$155.25 Add to cart View Product DetailsInterleukin-17A (IL-17A),also known as CTLA-8 and IL-17, is a proinflammatory cytokine belonging to the IL-17 family. It is secreted by Th17 cells, gamma/delta T cells, NK cells and neutrophils. IL-17A signals through IL-17 receptor A in a complex with receptor C or D to regulate NF-kappaB and MAP kinase activities. IL-17A plays important roles in the anti-microbial response and chronic inflammation. It stimulates the production of IL-6, IL-8 and G-CSF in epithelial and endothelial cells, and induces the expression of ICAM-1 in fibroblasts. Clinically, IL-17A has been associated with inflammatory diseases, such as rheumatoid arthritis, psoriasis and multiple sclerosis.
-

IL-17A, Mouse
$155.25 Add to cart View Product DetailsInterleukin-17A, (also known as CTLA-8) is a T cell-expressed pleiotropic cytokine that exhibits a high degree of homology to a protein encoded by the ORF13 gene of herpesvirus Saimiri. cDNA clones encoding IL-17 have been isolated from activated rat, mouse and human T cells. IL-17 represents a family of structurally-related cytokines that share a highly conserved C-terminal region but differ from one another in their N-terminal regions and in their distinct biological roles. The six known members of this family, IL-17A through IL-17F, are secreted as homodimers.
-

IL-18 BP, Human
$86.25 Add to cart View Product DetailsInterleukin-18 binding protein, also known as IL-18BP and tadekinig-alfa, is a secreted glycoprotein that contains an Ig-like C2-type domain. It is expressed in heart, lung, placenta and spleen. IL-18BP functions as an inhibitor of the proinflammatory cytokine IL-18. It binds to IL-18, prevents the binding of IL-18 to its receptor, and thus blocks IL-18-induced IFN-gamma production. The complete Ig domain has been shown to mediate the binding and neutralizing properties. IFN-gamma is able to upregulate the expression of IL-18BP, indicating that IL-18 activity is regulated by a feedback mechanism through IL-18BP.
-

IL-18, Rat
$306.19 Add to cart View Product DetailsInterleukin-18 (IL18, also known as interferon-gamma inducing factor or IGIF) is a cytokine that belongs to the IL-1 superfamily and is produced by macrophages and other cells. Its biological activity is pleiotropic and it has been shown to induce interferon-gamma production in KG-1 cells. The combination of this cytokine and IL12 has been shown to inhibit IL4 dependent IgE and IgG1 production, and enhance IgG2a production in B cells.
-

IL-19, Mouse
$340.69 Add to cart View Product DetailsInterleukin-19 (IL-19) is a cytokine belonging to the interleukin family. Structurally, IL-19 is grouped into the IL-10 sub-family, which also includes IL-20, IL-22, IL-24, and IL-26. In contrast to IL-10, which exists as a homodimer, IL-19 is stable and active as a monomer in vivo. IL-19 functions through the receptor complex composed of IL-20 Receptor α and IL-20 Receptor β, which is also utilized by IL-20 and IL-24. IL-19 is produced by active monocytes and stimulated synergistically by IL-17 and IL-13. The functions of IL-19 are to promote the development and function of Th2 cells and to enhance the production of Th2 cytokines. IL-19 is implicated in aging, vascular disease, Type I diabetes, and rheumatoid arthritis.
-
IL-1RA, Human
$254.44 Add to cart View Product DetailsInterleukin-1 receptor antagonist (IL-1ra) is a member of the IL-1 family. Endogenous IL-1ra is produced in numerous animal disease models as well as in human autoimmune and chronic inflammatory diseases. It binds to IL-1 receptors in competition with IL-1, but does not elicit intracellular response from this binding. Its role in counteracting the proinflammatory effects of IL-1 is being studied by numerous research groups. IL-4 and IL-13 have been shown to amplify the stimulatory effect of IL1-beta on the production of soluble and intracellular forms of IL1-ra. The regulated expression of IL1ra in various cell types has been shown to be influenced by cytokines. In synovial fibroblasts the synthesis of IL-1ra is markedly enhanced by IL-1, TNF-alpha, or PDGF.
-

IL-1α, Rat
$271.69 Add to cart View Product DetailsInterleukin-1 alpha (IL-1α), is produced in a variety of cells including monocytes, tissue macrophages, keratinocytes and other epithelial cells. Both IL-1 alpha and IL-1beta bind to the same receptor and have similar if not identical biological properties. These cytokines have a broad range of activities including stimulation of thymocyte proliferation via IL-2 release, B-cell maturation and proliferation, mitogenic FGF-like activity, and the ability to stimulate the release of prostaglandin and collagenase from synovial cells. However, whereas IL-1beta is a secreted cytokine, IL-1 alpha is predominantly a cell-associated cytokine.
-
IL-1β, Human
$340.69 Add to cart View Product DetailsInterleukin-1 beta (rhIL-1β) is a proinflammatory cytokine produced in a variety of cells including monocytes, tissue macrophages, keratinocytes and other epithelial cells. Both IL-1 alpha and IL-1 beta binds to the same receptor and has similar if not identical biological properties. These cytokines have a broad range of activities including, stimulation of thymocyte proliferation, by inducing IL-2 release, B-cell maturation and proliferation, mitogenic FGF-like activity and the ability to stimulate the release of prostaglandin and collagenase from synovial cells. However, whereas IL-1 beta is a secreted cytokine, IL-1 alpha is predominantly a cell-associated cytokine.
-

IL-1β, Human(CHO-expressed)
$194.06 Add to cart View Product DetailsInterleukin 1 beta is a proinflammatory cytokine produced in a variety of cells including monocytes, tissue macrophages, keratinocytes and other epithelial cells. Both IL-1 alpha and IL-1 beta binds to the same receptor and has similar if not identical biological properties. These cytokines have a broad range of activities including, stimulation of thymocyte proliferation, by inducing IL-2 release, B-cell maturation and proliferation, mitogenic FGF-like activity and the ability to stimulate the release of prostaglandin and collagenase from synovial cells. However, whereas IL-1 beta is a secreted cytokine, IL-1 alpha is predominantly a cell-associated cytokine.
-

IL-1β, Mouse
$340.69 Add to cart View Product DetailsInterleukin-1 Beta (IL-1β) is a proinflammatory cytokine produced in a variety of cells including monocytes, tissue macrophages, keratinocytes and other epithelial cells. Both IL-1 alpha and IL-1 beta binds to the same receptor and has similar if not identical biological properties. These cytokines have a broad range of activities including, stimulation of thymocyte proliferation, by inducing IL-2 release, B-cell maturation and proliferation, mitogenic FGF-like activity and the ability to stimulate the release of prostaglandin and collagenase from synovial cells. However, whereas IL-1 beta is a secreted cytokine, IL-1 alpha is predominantly a cell-associated cytokine.
-

IL-1β, Mouse(CHO-expressed)
$245.81 Add to cart View Product DetailsInterleukin-1 (IL-1) is a family of cytokines that play a central role in the regulation of immune and inflammatory responses to infections or sterile insults. IL-1α and IL-1β are the first two members discovered in this family, which are the products of distinct genes recognizing the same cell surface receptors. IL-1α and IL-1β are structurally related polypeptides that show approximately 25% homology at the amino acid level. Both proteins are produced by a wide variety of cells in response to stimuli such as those produced by inflammatory agents, infections, or microbial endotoxins. The proteins are synthesized as 31 kDa precursors that are subsequently cleaved into proteins with molecular weights of approximately 17.5 kDa. The specific protease responsible for the processing of IL-1β is interleukin 1β-converting enzyme (ICE)/caspase-1. Mature human and mouse IL-1β share approximately 75% amino acid sequence identity where human IL-1β has been found to be active on murine cell lines.
-
IL-1β, Rat
$271.69 Add to cart View Product DetailsInterleukin-1 Beta (IL-1 Beta) is a proinflammatory cytokine produced in a variety of cells including monocytes, tissue macrophages, keratinocytes and other epithelial cells. Both IL-1 alpha and IL-1 beta bind to the same receptor and have similar if not identical biological properties. These cytokines have a broad range of activities including, stimulation of thymocyte proliferation, by inducing IL-2 release, B-cell maturation and proliferation, mitogenic FGF-like activity and the ability to stimulate the release of prostaglandin and collagenase from synovial cells. However, whereas IL-1 beta is a secreted cytokine, IL-1 alpha is predominantly a cell-associated cytokine.
-

IL-1β, Rat
$521.81 Add to cart View Product DetailsInterleukin-1beta (IL-1β) is a non-secreted proinflammatory cytokine produced mainly by activated macrophages, as well as neutrophils, epithelial cells, and endothelial cells. It possesses metabolic, physiological, haematopoietic activities, and plays one of the central roles in the regulation of the immune responses. Both IL-1αand IL-1β binds to the same receptor and have similar but not identical biological properties. The mature rat IL1β shares 90 % a.a. sequence identity with cotton rat and mouse and 65 % to 77 % with canine, human,and rhesus IL1β.
-

IL-2 R α, His, Human
$146.63 Add to cart View Product DetailsInterleukin-2 receptor (IL-2R) is a heterotrimeric protein expressed on the surface of certain immune cells, such as lymphocytes, that binds and responds to the cytokine IL-2. The IL-2R is made up of 3 subunits – alpha (α), beta (β) and gamma (γ). The α and β chains are involved in binding IL-2, while signal transduction following cytokine interaction is carried out by the γ-chain, along with the β subunit. The β and γ chains of the IL-2R are members of the type I cytokine receptor family. IL-2R has a high binding affinity to IL-2 and is expressed by antigen-activated T lymphocytes (T cells). IL-2 Rα is also known as CD25, p55, and Tac (activated T cell) antigen.
-
IL-2, Human
$150.94 Add to cart View Product DetailsInterleukin-2 (IL-2) is a Oglycosylated, four α-helix bundle cytokine that has potent stimulatory activity for antigen-activated T cells. It is expressed by CD4+ and CD8+ T cells, γδ T cells, B cells, dendritic cells, and eosinophils. IL-2/IL-2R signaling is required for T-cell proliferation and other fundamental functions which are essential for the immune response. IL-2 stimulates growth and differentiation of B-cells, NK cells, lymphokine activated killer cells, monocytes, macrophages and oligodendrocytes.
-

IL-2, Human
$86.25 Add to cart View Product DetailsInterleukin-2 (IL-2) is a Oglycosylated, four α-helix bundle cytokine that has potent stimulatory activity for antigen-activated T cells. It is expressed by CD4+ and CD8+ T cells, γδ T cells, B cells, dendritic cells, and eosinophils. IL-2/IL-2R signaling is required for T-cell proliferation and other fundamental functions which are essential for the immune response. IL-2 stimulates growth and differentiation of B-cells, NK cells, lymphokine activated killer cells, monocytes, macrophages and oligodendrocytes.
-

IL-21, Human
$271.69 Add to cart View Product DetailsInterleukin-21 (IL-21) belongs to the Type I four helix bundle cytokines, and shares the common cytokine receptor γ chain with IL-2, IL-4, IL-7, IL-9, and IL-15. IL-21 is expressed by CD4+ T cells, natural killer (NK) T cells, and Th17 cells, and the IL-21 receptor is highly expressed on CD4+ and CD8+ B cells; indeed, IL-21 plays a pivotal role in the survival and proliferation of B cells, and their differentiation to immunoglobulin (Ig) producing cells. IL-21 up-regulates and down-regulates the production of IgG1 and IgE by B cells, respectively, and diminishes the severity of allergy and asthma. In some case, IL-21 also induces the apoptosis of B cells. The other roles of IL-21 include regulation of innate immune systems, implication on autoimmunity, and antitumor actions.
-

IL-21, Mouse
$276.00 Add to cart View Product DetailsInterleukin-21 (IL-21) belongs to the Type I four helix bundle cytokines, and shares the common cytokine receptor γ chain with IL-2, IL-4, IL-7, IL-9, and IL-15. IL-21 is expressed by CD4+ T cells, natural killer (NK) T cells, and Th17 cells. The IL-21 receptor is highly expressed on CD4+ and CD8+ B cells. IL-21 plays a pivotal role in the survival and proliferation of B cells, and their differentiation to immunoglobulin (Ig) producing cells. IL-21regulates the production of IgG1 and IgE by B cells, and diminishes the severity of allergy and asthma. In some cases, IL-21 induces B cell apoptosis. Other roles of IL-21 include regulation of the innate immune system, implication in autoimmunity, and antitumor activity.
-

IL-22, Human
$276.00 Add to cart View Product DetailsInterleukin-22(IL-22) belongs to a group of cytokines called the IL-10 family or IL-10 superfamily (including IL-19, IL-20, IL-24, and IL-26) which are a class of potent mediators of cellular inflammatory responses. It shares use of IL-10R2 in cell signaling with other members of this family, such as IL-10, IL-26, IL-28A/B and IL-29. IL-22 is produced by activated DC and T cells and initiates innate immune responses against bacterial pathogens in epithelial cells such as those in the lung and gut. IL-22 along with IL-17 is produced by splenic LTi-like cells and Th17 cells and likely plays a role in the coordinated response of both adaptive and innate immune systems.IL-22 signals through a receptor system consisting of IL-10R-β/CRF2-4 and IL-22R, both of which are members of the class II cytokine-receptor family.
-

IL-22, Human(HEK 293-expressed)
$271.69 Add to cart View Product DetailsInterleukin-22(IL-22) belongs to a group of cytokines called the IL-10 family or IL-10 superfamily (including IL-19, IL-20, IL-24, and IL-26) which are a class of potent mediators of cellular inflammatory responses. It shares use of IL-10R2 in cell signaling with other members of this family, such as IL-10, IL-26, IL-28A/B and IL-29. IL-22 is produced by activated DC and T cells and initiates innate immune responses against bacterial pathogens in epithelial cells such as those in the lung and gut. IL-22 along with IL-17 is produced by splenic LTi-like cells and Th17 cells and likely plays a role in the coordinated response of both adaptive and innate immune systems.IL-22 signals through a receptor system consisting of IL-10R-β/CRF2-4 and IL-22R, both of which are members of the class II cytokine-receptor family.






