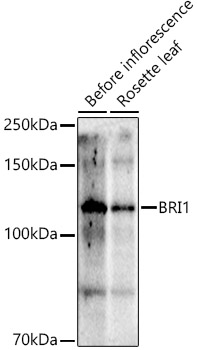
Description
Encodes a plasma membrane localized leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. BRI1 ligand is brassinolide which binds at the extracellular domain. Binding results in phosphorylation of the kinase domain which activates the BRI1 protein leading to BR responses. Residue T-1049 and either S-1044 or T-1045 were essential for kinase function in vitro and normal BRI1 signaling in planta. The structure of BRI1 ligand-binding domain has been determined at 2.5A resolution. Although BAK1 and BRI1 alone localize in the plasma membrane, when BAK1 and BRI1 are coexpressed, the heterodimer BAK1/BRI1 they form is localized in the endosome. BRI1 appears to be involved in the autonomous pathway that regulates the transition to flowering, primarily through its effects on FLC expression levels, as uncovered by double mutant analyses. This most likely occurs as a result of BRI1-dependent effects on histone acetylation, but not histone triMeH3K4 methylation, at the FLC locus.