Description
Size quantity : 1-EA
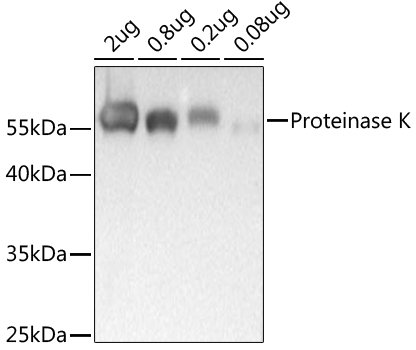
Description : Proteinase K is an endopeptidase belonging to the subtilisin group of serine proteases. Proteinase K was isolated from the mold (Parengyodontium album or previously known as Tritirachium album). Proteinase K has five cysteines. Four of the cysteine residues form two disulfide bonds (34-124 and 179-248, respectively) and one lies below one of the catalytic triads. Proteinase K often binds two calcium ions required for full enzymatic activity. In molecular biology laboratories, proteinase K is useful during preparation of DNA or RNA samples by degrading and inactivating proteins.