Description
Sucrose, (Beet Derived), Low Endotoxin, USP/NF, EP, JP, ChP
BioHale high purity and low endotoxin excipients from DFE Pharma facilitate the stabilization of biomolecules, reducing the loss of activity and improving efficacy.
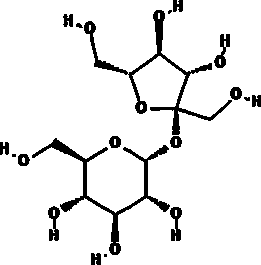
BioHale Sucrose is a non-reducing crystalline disaccharide made up of glucose and fructose. It is well suited to provide solution-state stabilization, as well as cryo and lyo-protection for biomolecules to be used in various administration forms including parenteral or ophthalmic.
Stabilizing agent
BioHale Sucrose is a non-reducing sugar and does not react with amino acids or proteins, inhibiting the Maillard reaction. BioHale Sucrose provides solution-state stabilization to fragile biomolecules.
Cryo- and lyoprotectant
BioHale Sucrose, a high purity disaccharide excipient, protects the biologic drugs from the freeze-related (cryoprotectant) and drying related (lyoprotectant) stresses. This makes BioHale Sucrose particularly suitable in the stabilization process of today’s biologics.
Product Information
• Description: White, or almost white, crystalline powder, or lustrous, colorless or white, or almost white, crystals
• Multi-compendial specifications compliant with USP-NF, JP, ChP monographs
• Produced in state-of-the-art, FDA-inspected manufacturing facilities in accordance with ICH Q7, which provides cGMP guidance for the manufacturing of APIs
• Active purification steps ensure excipients are independent of raw material variation
• Source: Plant derived, isolated from sugar beet
• Molecular Formula: C12H22O11
• Molecular Weight: 342.30 g/mol
• CAS Number: 57-50-1
• Tg: ~61°C
Product Specifications
• Endotoxin: ≤ 0.25 EU/g
• Heavy Metals: ≤ 5 ppm
• Elemental Impurities: Complies with ICH Q3D
• Total Impurities: ≤ 2.0%
• Reducing Sugars: ≤ 0.07%